Autologous Cell Therapy
EXG-34217 and Telomere Biology Disorders with Bone Marrow Failure
EXG-34217 is an autologous cell therapy for telomere biology disorders with bone marrow failure that uses Elixirgen Therapeutics’ proprietary ZSCAN4 technology to extend the telomeres of the patients. Elixirgen Therapeutics is currently conducting a Phase I/II, open label, single center clinical trial to assess the safety and tolerability of EXG-34217 at Cincinnati Children’s Hospital Medical Center. ClinicalTrials.gov ID: NCT04211714
EXG-34217’s process was designed for the most safety and least burden possible to the patients. First, the patients’ CD34+ hematopoietic stem cells are mobilized, and then collected using standard apheresis. Next, the CD34+ cells are treated with a human ZSCAN4 protein-expressing controllable RNA vector in a closed, sterile tubing system outside the patient’s body for 24 hours. Finally, those cells are re-infused back to the patient. The process does not require pre-conditioning of the bone marrow.

EXG-34217 uses Elixirgen Therapeutics’ proprietary ZSCAN4 technology to extend the telomeres of the patients’ cells.
What is ZSCAN4?
ZSCAN4 (zinc finger and SCAN domain containing 4) was originally identified by Minoru Ko’s lab at the National Institutes of Health (NIH) as a gene expressed specifically at the 2-cell stage of mouse preimplantation embryos (Falco et al., 2007). In addition to the work at the NIH, further studies by Elixirgen Therapeutics have demonstrated the functions of ZSCAN4 in humans. The ZSCAN4 protein contains a SCAN domain for putative protein-protein interactions as well as four zinc finger domains for putative DNA binding. ZSCAN4 functions to enhance genome stability and elongate telomeres, repair chromosome abnormalities, and restore the developmental potential of stem cells.
EXG-34217 uses Elixirgen Therapeutics’ proprietary ZSCAN4 technology to extend the telomeres of the patients’ cells.
Future use of ex vivo controllable RNA
Elixirgen Therapeutics is planning to expand the ex vivo controllable RNA to additional indications involving autologous cell therapy, such as use in CAR-T Cell Therapy.
- Zscan4: a novel gene expressed exclusively in late 2-cell embryos and embryonic stem cells. Falco G, Lee SL, Stanghellini I, Bassey UC, Hamatani T, Ko MS. Dev Biol. 2007 Jul 15;307(2):539-50. [PMID:17553482][http://www.sciencedirect.com/science/article/pii/S0012160607008792]
- An in situ hybridization-based screen for heterogeneously expressed genes in mouse ES cells. Carter MG, Stagg CA, Falco G, Yoshikawa T, Bassey UC, Aiba K, Sharova LV, Shaik N, Ko MS. Gene Expr Patterns. 2008 Feb;8(3):181-98. [PMID:18178135][https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2238805/]
- Zscan4 regulates telomere elongation and genomic stability in ES cells. Zalzman M, Falco G, Sharova LV, Nishiyama A, Thomas M, Lee SL, Stagg CA, Hoang HG, Yang HT, Indig FE, Wersto RP, Ko MS. Nature. 2010 Apr 8;464(7290):858-63. [PMID:20336070][https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2851843/]
- Zscan4 transiently reactivates early embryonic genes during the generation of induced pluripotent stem cells. Hirata T, Amano T, Nakatake Y, Amano M, Piao Y, Hoang HG, Ko MS. Sci Rep. 2012;2:208. [PMID:22355722][https://www.nature.com/articles/srep00208]
- Inflammation increases cells expressing ZSCAN4 and progenitor cell markers in the adult pancreas. Ko SB, Azuma S, Yokoyama Y, Yamamoto A, Kyokane K, Niida S, Ishiguro H, Ko MS. Am J Physiol Gastrointest Liver Physiol. 2013 Jun 15;304(12):G1103-16. [PMID:23599043][http://ajpgi.physiology.org/content/304/12/G1103.long]
- Repression of global protein synthesis by Eif1a-like genes that are expressed specifically in the two-cell embryos and the transient Zscan4-positive state of embryonic stem cells. Hung SS, Wong RC, Sharov AA, Nakatake Y, Yu H, Ko MS. DNA Res. 2013 Aug;20(4):391-402. [PMID:23649898][https://academic.oup.com/dnaresearch/article-lookup/doi/10.1093/dnares/dst018]
- Zscan4 restores the developmental potency of embryonic stem cells. Amano T, Hirata T, Falco G, Monti M, Sharova LV, Amano M, Sheer S, Hoang HG, Piao Y, Stagg CA, Yamamizu K, Akiyama T, Ko MS. Nat Commun. 2013;4:1966. [PMID:23739662][https://www.nature.com/articles/ncomms2966]
- Correction of Down syndrome and Edwards syndrome aneuploidies in human cell cultures. Amano T, Jeffries E, Amano M, Ko AC, Yu H, Ko MS. DNA Res. 2015 Oct;22(5):331-42. [PMID:26324424][https://academic.oup.com/dnaresearch/article-lookup/doi/10.1093/dnares/dsv016]
- Transient bursts of Zscan4 expression are accompanied by the rapid derepression of heterochromatin in mouse embryonic stem cells. Akiyama T, Xin L, Oda M, Sharov AA, Amano M, Piao Y, Cadet JS, Dudekula DB, Qian Y, Wang W, Ko SB, Ko MS. DNA Res. 2015 Oct;22(5):307-18. [PMID:26324425][https://academic.oup.com/dnaresearch/article-lookup/doi/10.1093/dnares/dsv013]
- Emergence of undifferentiated colonies from mouse embryonic stem cells undergoing differentiation by retinoic acid treatment. Sharova LV, Sharov AA, Piao Y, Stagg CA, Amano T, Qian Y, Dudekula D, Schlessinger D, Ko MS. In Vitro Cell Dev Biol Anim. 2016 May;52(5):616-24. [PMID:27130680][https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4884469/]
- Zygotic Genome Activation Revisited: Looking Through the Expression and Function of Zscan4. Ko MS. Curr Top Dev Biol. 2016;120:103-24. [PMID:27475850][http://www.sciencedirect.com/science/article/pii/S0070215316301016]
- Expression analysis of the endogenous Zscan4 locus and its coding proteins in mouse ES cells and preimplantation embryos. Ishiguro KI, Nakatake Y, Chikazawa-Nohtomi N, Kimura H, Akiyama T, Oda M, Ko SB, Ko MS. In Vitro Cell Dev Biol Anim. 2017 Feb;53(2):179-190. [PMID:27699651][https://link.springer.com/article/10.1007%2Fs11626-016-0097-y]
- Zscan4 is expressed specifically during late meiotic prophase in both spermatogenesis and oogenesis. Ishiguro KI, Monti M, Akiyama T, Kimura H, Chikazawa-Nohtomi N, Sakota M, Sato S, Redi CA, Ko SB, Ko MS. In Vitro Cell Dev Biol Anim. 2017 Feb;53(2):167-178. [PMID:27699653][https://link.springer.com/article/10.1007%2Fs11626-016-0096-z]
Immunotherapy and Vaccines
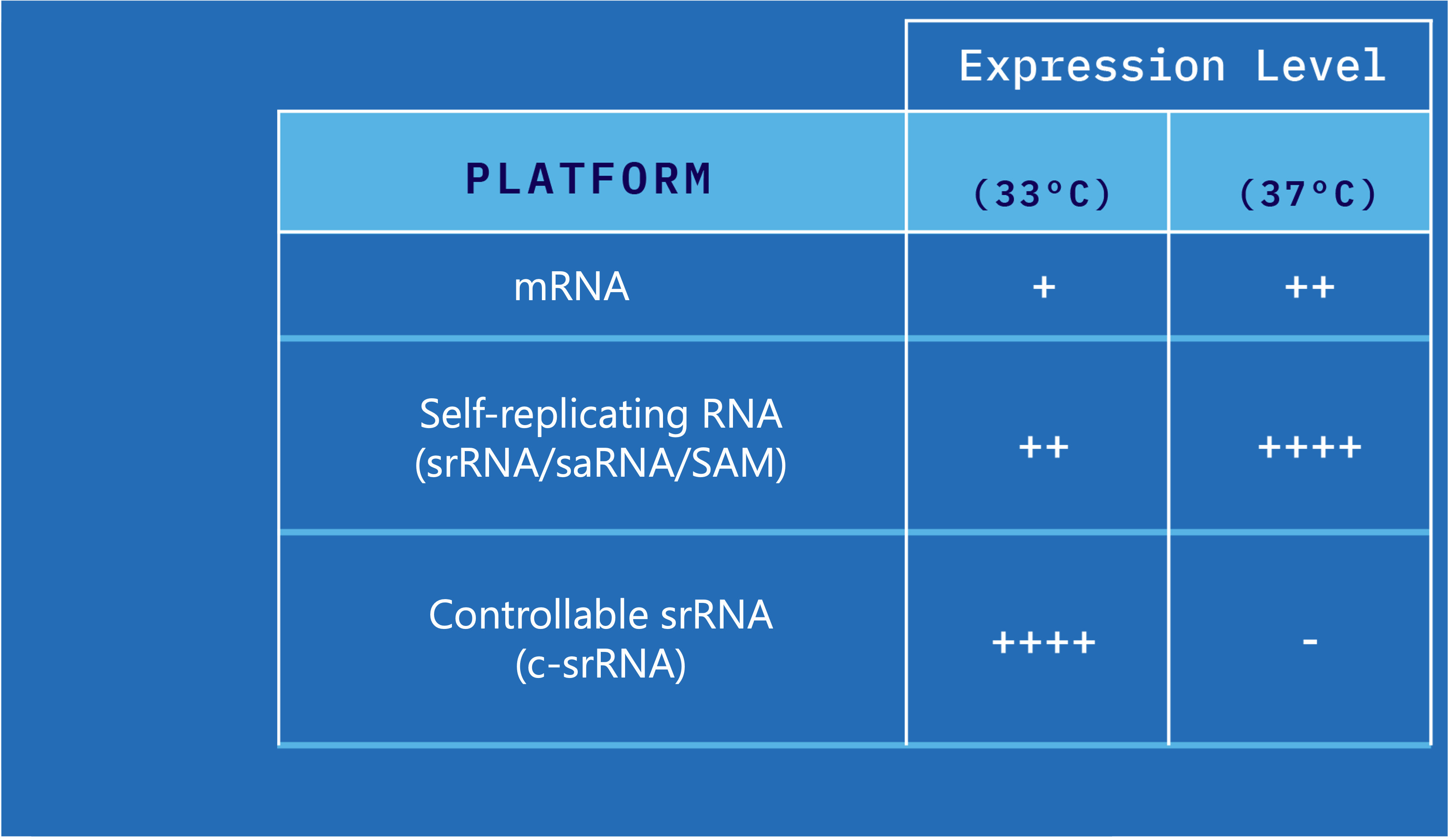
Controllable self-replicating RNA (c-srRNA) can be directly injected for use as a cancer immunotherapy or vaccine. in vivo c-srRNA is temperature-controllable, intradermally-injected, srRNA designed with unique safety and efficacy benefits.
-
1.
Temperature Control
c-srRNA is active at normal skin temperature (where it is injected, intradermally) but inactive at normal core body temperature. This improves the safety of the product by ensuring that the c-srRNA is only active at the injection site, serving as an off-switch and a mechanism to reduce non-local expression.
-
2.
Optimization for 30°C-35°C
Not only is in vivo c-srRNA active at 30°C-35°C, it is also optimized for better expression at that temperature range.